Imeka: The Gold Standard of Clinical White Matter Imaging
Our technology is
powerful, unique, and non-invasive.
Imeka + you
Imeka’s
people
Webinars
IMEKA: Because imaging means so much more with powerful processing tools
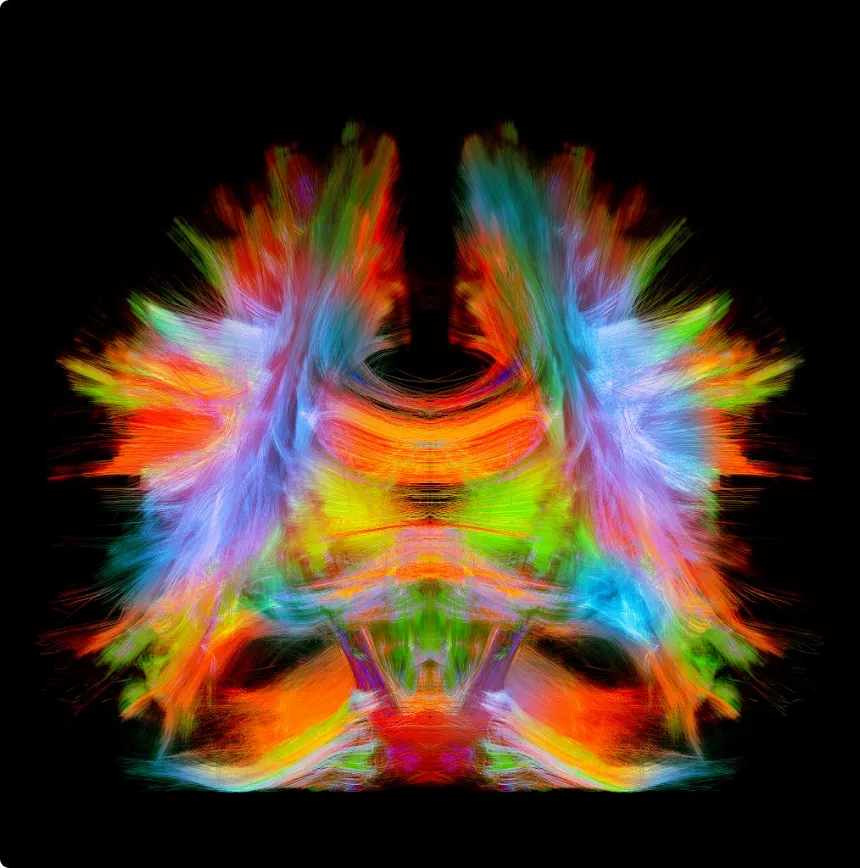
Advanced white matter imaging—such as diffusion MRI (dMRI)—is a readily available technology that picks up microstructural properties of the central nervous system. Data can be collected in as little as five minutes on a standard MRI machine.
Imeka’s ANDI (Advanced Neuro Diagnostic Imaging) clinical Tool combines white matter imaging with artificial intelligence to analyze white matter MRI signals in greater detail than any tool available for clinicians.
IMEKA’S PEOPLE
The Imeka team is made of some of the most dedicated, driven, science-minded people you’ll ever meet.

EXECUTIVE TEAM




Jean-René Bélanger
Jean-René is Imeka’s rock. He drives the company vision. Since starting at Imeka in 2011, Jean-René has driven consistent growth, established strategic partnerships with the largest companies working in neurology, secured significant investments, and built a team with extraordinary synergy that laid the foundations for Imeka’s credibility in the scientific world. He managed a new office in Boston, MA, and launched a strong marketing strategy in the USA.
Jean-René’s work as CEO has provided the team with a transformational vision and continuous income streams so they can focus on doing what they do best: contributing to finding treatments for neurodegenerative diseases.
Valérie Lacroix
Valérie is Imeka’s driving force. She makes the company vision come true. Valérie has been working in the pharmaceutical and life sciences fields since 2004, and now she is shaping the future of Imeka.
As Executive Vice-President of Imeka, Valérie is involved in every part of the company, from overseeing and harmoniously integrating major functions of the business to recruiting stellar new talent and ensuring smart, sustainable growth. Valérie’s passion, vision, experience, and human touch add immeasurable value to Imeka’s culture and offerings.
Prior to working at Imeka, Valerie held senior roles in business development, corporate strategy, sales, and operations.
Jean-Christophe Houde
Jean-Christophe has a bachelor’s and a master’s degree in computer science with a specialization in image analysis. He is in charge of managing the operations unit at Imeka and ensures Imeka stays at the forefront of the field by keeping up to date on developments in the field of medical imaging analysis. His in-depth knowledge of dMRI and hands-on experience come from his role as a research associate at the Sherbrooke Connectivity Imaging Lab (SCIL), which is headed by Maxime Descoteaux (Imeka’s co-founder and chief science officer).
Luc Champagne
ADVISORS





Dr. Lawrence Tanenbaum
Dr. Tanenbaum is the Vice President, Chief Technology Officer and Director of Advanced Imaging at Radnet Inc. (since 2015), having come from Icahn School of Medicine at Mount Sinai in New York where he attended in Neuroradiology and served as an Associate Professor of Radiology, Director of MRI, CT and Outpatient / Advanced Imaging Development since 2008. Prior to that he spent over 20 years in the private practice of Radiology at the JFK Medical Center / New Jersey Neuroscience Institute as Director of MRI, CT and Neuroradiology.
Dr. Tanenbaum is a senior member of the American Society of Neuroradiology, and long-term member of the Radiological Society of North America. He is a past President of the Eastern Society of Neuroradiology, and the national Clinical Magnetic Resonance Imaging Society and former Editor in Chief of their Journal Vision. He is a member of the Roster of Distinguished Scientific Advisors of the RSNA as well as several panels and committees of the American College of Radiology including the Expert Panel on Neuroimaging and the CPI / Neuroradiology Expert Review Panel. Dr. Tanenbaum is a member of the editorial boards of several journals and educational organizations and is a columnist and Associate Editor for Artificial Intelligence of Applied Radiology.
Dr. Tanenbaum is a long-term collaborator with the medical imaging industry and chairs several advisory boards (OEM, pharma, and AI). He has interests in developing applications of AI and machine learning, contrast agents, MR, CT and advanced rendering. Dr. Tanenbaum is passionate about advancing the clinical practice of medicine focusing on patient centric care, efficiency, radiation dose and physiologic imaging. He is an active educator with interests in advanced imaging and innovative value-adding applications in the spine and brain. He has authored over 100 scholarly and peer reviewed articles which have been cited over 1300 times, continues to chair educational and academic meetings and has delivered close to 2000 invited lectures around the world.
Richard Macary
Rich Macary is the President of Sinaptica Therapeutics, a clinical-stage electromagnetic therapeutics company developing a novel, noninvasive closed loop neuromodulation approach for the treatment of Alzheimer’s disease. As one of the company’s co-founders, Rich helped drive product development and partnering efforts as well as securing FDA Breakthrough Device Designation for the company’s proprietary SinaptiStimTM- AD System. Prior to his current role, Rich was a consultant, strategic advisor and a senior executive at Sarepta Therapeutics, a global biotechnology company and leader in RNA based therapeutics and genetic medicines targeting rare diseases. In his role as Vice President of Business Development, Rich focused on developing new opportunities and therapeutic targets for the company’s RNA technologies as well as complimentary technologies and related IP. Prior to and post his roles at Sarepta, Rich held several senior executive roles at Delos, a wellness real estate and technology company, where he lead business development, strategic investments and overall strategy as both President of Delos Ventures and Chief Strategy Officer. In his roles at Delos, Rich achieved a number of key accomplishments including conceiving, developing and launching the Well Living Lab, a Delos and Mayo Clinic collaboration to study the impact of indoor environments on the health and well-being of building occupants.
Rich spent the prior 15 years as a corporate consultant, advisor and analyst to both institutional and high-net-worth investors as well as a consultant, advisor, investor and board member to several public and private early to mid-stage companies operating in a diverse range of industries including biotechnology, medical devices, software, fintech and consumer products among others. Rich currently holds several board positions and remains a managing partner of Macary Advisors, a biotech/medtech consulting and advisory firm currently engaged with several promising companies within the fields of diagnostics, therapeutics, medical devices and digital health. His research and opinions have appeared in many media outlets including Forbes, The Wall Street Journal, Wired, CNN, Barron’s, Reuters and Bloomberg.
Michael Davis, MD, FACS, FRCS(HON) Colonel (ret), USAF, MC
Dr. Davis received his medical doctorate (MD) from the Uniformed Services University with post-
graduate education/ board certification in General Surgery as well as Reconstructive Surgery (UT Health,
San Antonio | University of Alabama at Birmingham); BA, Physiology and Cell Biology, University of
California, Santa Barbara.
Colonel (ret) Michael Davis, is recently retired after 26 years of military service, and has accumulated
an extensive, rare knowledge base involving the clinical, scientific, operational and US
governmental programmatic domains. He has garnered numerous military and medical publications
and awards, and serves as a reviewer for professional journals. He is a Fellow of the American College
of Surgeons (FACS) as well as an Honorary Fellow of the Royal College of Surgeons (England).
Colonel (ret) Davis has served in escalating Department of Defense programmatic roles as Deputy
Commander, US Army Institute of Surgical Research, then culminating as Director, US Combat
Casualty Care Research Program- the Department’s largest medical R&D program.
While deployed to Afghanistan in support of Operation Enduring Freedom from 2009-2010, Michael
served as Chief of Reconstructive Surgery. Additionally, he founded and directed the RESTOR
(Restorative Endeavor for Service-members Through Optimization of Reconstruction) Research
Program for advanced reconstructive surgery and regenerative medicine research for injured warfighters.
With more than 30 years in the clinical and biomedical science field, Colonel (ret) Davis provides services
and expertise in Trauma, Regenerative/ Restorative Medicine, Federal Government Relations, Defense
Health, Cyber and National Security. He has garnered expertise in contemporary and emerging
Department of Defense operational concepts and maintains a deep understanding of working within the
Department of Defense, Defense Health Agency, and Army/ Navy/ Air Force/ Marine Corps Service
specific programmatics as well as with International allies. Colonel (ret) Davis also founded and serves
as CEO of MD3 Multidimensional Medical Consulting.
Maxime Descoteaux
Maxime received a PhD from Inria Sophia-Antipolis and did a postdoctoral fellowship in computer science and neuroimaging at NeuroSpin, CEA Saclay in Paris. He is a world-renowned expert in advanced white matter imaging and medical imaging. Maxime supervises the science, research, and software developments at Imeka.
In addition to his work with Imeka, Maxime leads the Sherbrooke Connectivity Imaging Lab (SCIL), known for its integrated approach to advanced white matter imaging. Maxime’s credentials include:
- 9695 citations; 125+ journal papers; h-index 48, i10-index 131
- Member of the Royal Society of Canada’s College of New Scholars, Artists, and Scientists
- Full professor, Computer Science, University of Sherbrooke, QC, Canada
- Leads a lab of 15 members (MSc, PhD, postdocs)
- International leader and conference/workshop organizer in diffusion MRI
Pierre-Marc Jodoin
Pierre-Marc is a world expert in computer vision and deep learning. He received a PhD in computer vision from the University of Montreal, followed by a postdoc at Boston University. He is currently a full professor at the Université de Sherbrooke, working on the development of deep learning solutions to various digital imaging problems. Pierre-Marc supervises software development at Imeka.
In addition to his work with Imeka, Pierre-Marc runs the Videos & Images Theory and Analytics Laboratory (VITAL), which is 100% focused on deep learning–based techniques. VITAL focuses on researching state-of-the-art algorithms in image processing, computer vision, video analytics, medical imaging, and statistical models.
WEBINARS
Between 2020 and 2023, we invited great scientists and collaborators to discuss imaging, the brain and different pathologies. It was a great way to understand how all of these come together for better outcomes for patients.

All our webinars are available on our YouTube channel. Click a title to watch.
Alexa Pichet Binette, PhD, postdoctoral fellow at Lund University, Sweden
Guido Guberman, MD/PhD candidate at McGill University
Concussion Biomarkers: Novel Imaging and Advanced Statistics
Sami Obaid, MD, CM, FRCSC, Neurosurgeon, PhD candidate, University of Montreal
Utility of Tractography and Connectomics in Neurosurgery : Why Tracking Matters
Ann-Marie Beaudoin, MD, Neurology Department,
Centre hospitalier universitaire de Sherbrooke,
Université de Sherbrooke
Tim Sinnecker, neurologist at the University Hospital of Basel, and postdoctoral researcher at the Medical Image Analysis Center (MIAC) in Basel, Switzerland
Quantitative MRI to Assess Tissue Damage in Multiple Sclerosis
Julien Cohen-Adad, PhD and Associate Professor, Polytechnique Montreal
François Rheault, PhD in Neuroimaging from the SCIL
A Brain Odyssey: Challenges and Solutions for Tractography in Clinical Settings
Maggie Roy, PhD, Research Center on Aging, SCIL
The Role of White Matter in Alzheimer’s Disease: New Insights from Tractography and PET Imaging
Kevin Whittingstall, PhD and Professor,
University of Sherbrooke
Visualizing Blood Vessel Structure and Function in the Human Brain
Pierre-Marc Jodoin, PhD, Imeka’s co-founder and Chief Artificial Intelligence Officer
AI: What’s Under the Hood? Why & How Does It Work?
Maxime Descoteaux, PhD, Imeka’s co-founder and Chief Science Officer
Diffusion MRI to Map White Matter Integrity and Assess Neuroinflammation, Demyelination and Axonal Loss
Let’s work together!
Like you, our main goal is to play an influential role—and minimize risk—in the search for and development of cures for brain diseases. Let’s see what we can do together.








