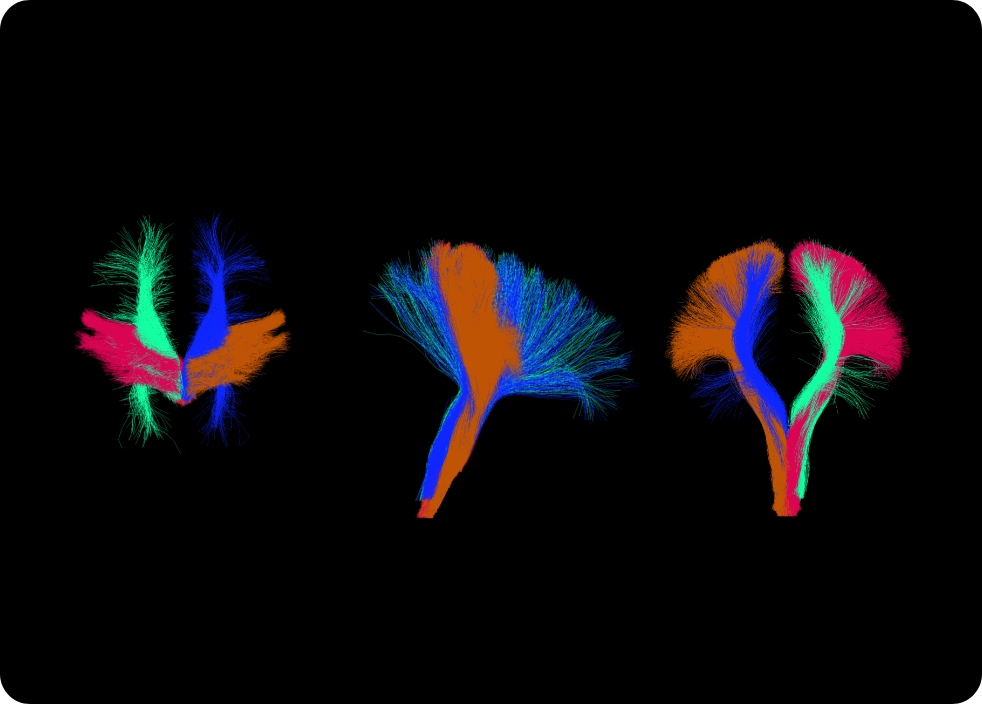Imeka at RSNA 2025 – Visit booth #5644
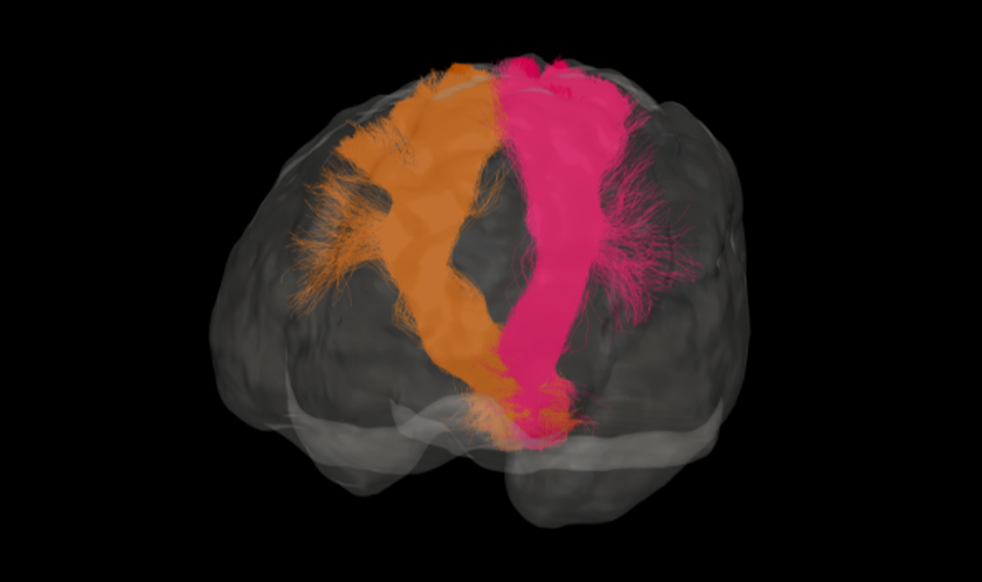
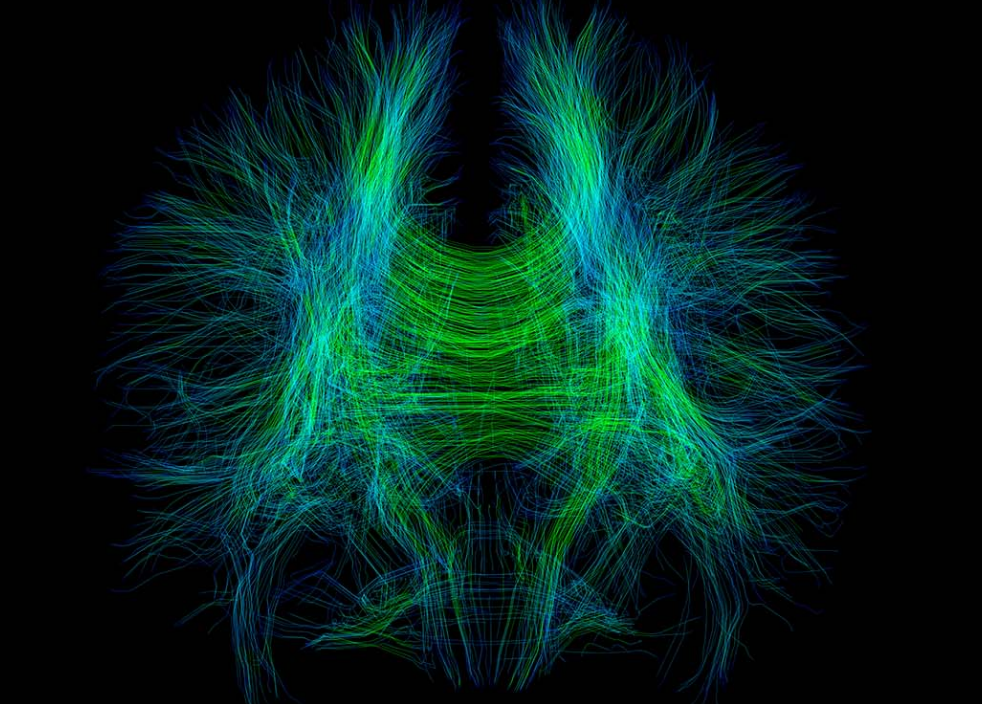
We’ll be showcasing our flagship product – ANDIAdvanced Neuro Diagnostic Imaging – at this year’s RSNA in Chicago, at McCormick Place, in the south Hall!
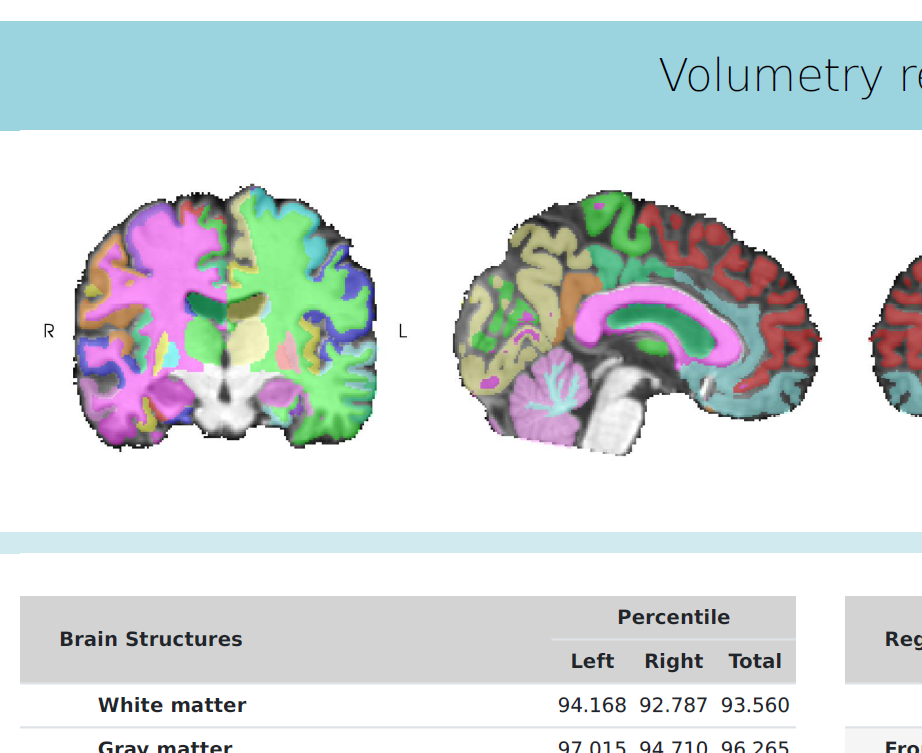
We are very excited to showcase ANDIAdvanced Neuro Diagnostic Imaging 2.0 during this years event, adding brain volumetry to ANDI’s capabilities.